The European partnership on One Health Antimicrobial Resistance (EUP OHAMR) is one of the key partnerships that has been identified by the European Commission within the framework of the Horizon Europe programme to support Resarch & Innovation to respond to the challenges of antimicrobial resistance. The first call and launch of other activities of this joint research programme between the European Commission and Member States are planned to take place in 2025.
In the research framework of Horizon Europe, the European Commission, in collaboration with the member states, has identified 49 new candidate European Partnerships of which, one of them is the “One Health AMR Partnership” (EUP OHAMR). The EUP OHAMR will be a partnership co-funded by EU countries and the European Commission (EC), with research funders and ministries at the core of the consortium. A Strategic Research and Innovation Agenda (SRIA) will be implemented through joint calls and additional activities. The main goal is to contribute to achieving the objectives of the European One Health Action Plan against AMR and the WHO Global Action Plan on AMR, both aimed at reducing the threat of antimicrobial resistance (AMR).
The EUP OHAMR aims to coordinate and align AMR activities and funding between countries as well as with the Commission. It will also facilitate national coherence between different services and ministries with responsibility for the various aspects of AMR (e.g. human health, agriculture, environment, industry, finances).
The new partnership builds on the work of the JPIAMR, but has a bigger ambition, as well as a stronger integration of social sciences and humanities, support of innovation, and international engagement to address the challenges of AMR.
In the proposal that was submitted in September 2024 to the EC, the EUP OHAMR consortium consists of 51 organisations from 28 countries that together with the EC will commit over 340 MEUR over 10 years to boost One Health AMR R&I in all its dimensions: coordinating joint transnational calls, actions to strengthen the capacity of the AMR research community, provide frameworks for alignment of policy and stakeholder engagement, and tools to valorise the new knowledge for the benefit of citizens and society as a whole.
Parties involved
The core group of formal members and signatories will comprise relevant ministries, funding agencies and other organisations from the participating countries.
- Countries: EU Member States and Associated Countries funding agencies
- European Commission lead: DG Research and Innovation – Combating Diseases
Expected Impact
- Novel solutions to prevent and treat infectious diseases affected by AMR, improved diagnosis and control of the spread of resistant microorganisms, testing and validation of such solutions and facilitating their uptake or implementation.
- Decreased burden of infectious diseases, notably due antimicrobial resistant pathogens and progress towards Sustainable Development Goal No. 3 ‘Ensure healthy lives and promote well-being for all at all ages’.
- Closed knowledge gaps on AMR (including those identified in the European One Health Action Plan against AMR and the EU Strategic Approach to Pharmaceuticals in the Environment), support provided to regulatory science and inform policymaking.
The Strategic Research and Innovation Agenda, SRIA
The strategic foundation of the EUP OHAMR is outlined in the Strategic Research and Innovation Agenda (SRIA), which covers research on bacteria, viruses, fungi, and parasites and resistance in pathogens that are transmitted among humans or between animals and humans, and present in the environment including wildlife having consequences on human health.
The EUP OHAMR SRIA has been developed by the Coordination and Support Action DESIGN OH AMR, in close collaboration with the JPIAMR Scientific Advisory Board and other experts, the JPIAMR member states, countries who expressed their interest to join the EUP OHAMR, and other stakeholders.
A first draft was published in May 2023 and the current updated version was published in September 2024.
An important element of the SRIA are the Research and Innovation (R&I) Objectives. To get a full understanding of the R&I objectives, please consult the long version where each R&I objective is described in detail.
The Research and Innovation Objectives are structured around five thematic areas and includes several cross-cutting themes.
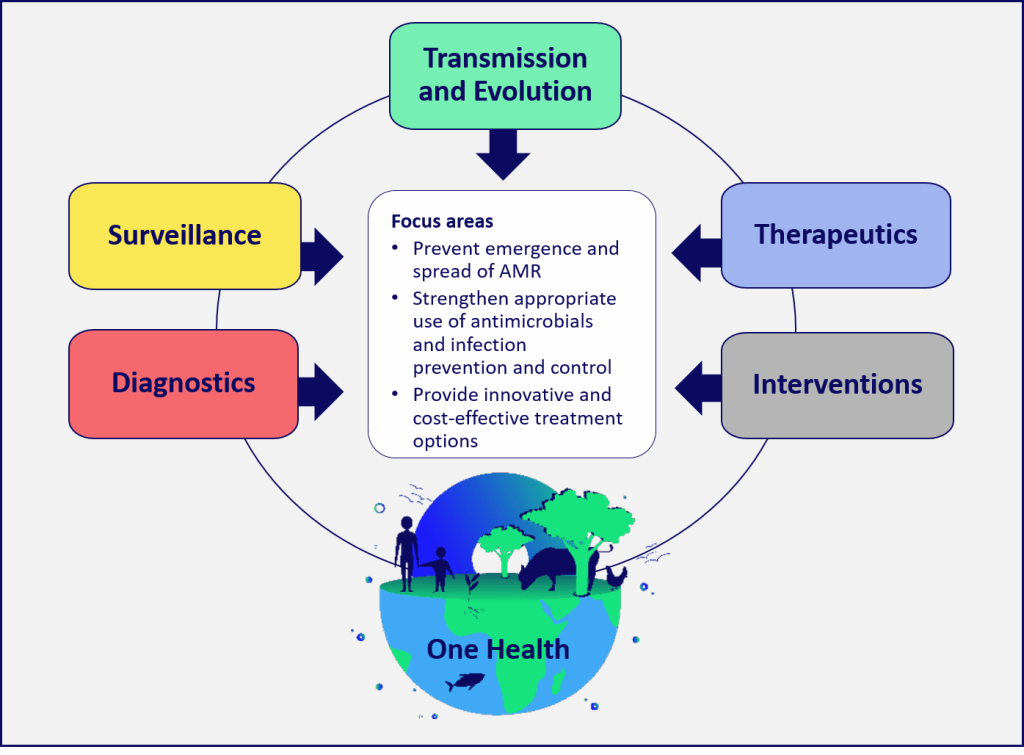
To read the reports from the consultations, please visit the documents libary page.
The Roadmap of Actions
The draft Roadmap of Actions has been developed by DESIGN OH AMR partners, future potential partners of OHAMR and additional experts and through several consultations with stakeholders.
Timeline
- 2021-2023: Identification of research and innovation objectives and definition of Partnership objectives and Roadmap of joint actions.
- 2022-2023: Collection of Members States commitments
- 2024: SRIA and proposal submission to the European Commission
- 2025: Launch of first call and start of activities
To stay updated about the progress of the OHAMR Partnership please subscribe to the JPIAMR newsletter.
Further information and contact
Contact JPIAMR: ohamr@vr.se
Contact Commission services: RTD-COMBATTING-DISEASES@ec.europa.eu
